Randomize Participants for Multi-Arm Studies
Randomized Controlled Trials (RCTs) are crucial in clinical research. This guide shows two ways to randomly assign participants to different study arms or groups within Avicenna, even though it's not a built-in feature yet.
The Situation
You are running an RCT and need to randomize participants into different arms (e.g., control vs. intervention). Avicenna currently lacks a dedicated randomization feature. You need a workaround to achieve this randomization directly within the platform or by integrating an external process.
The methods described here can also generate a random value as a survey response for other purposes.
What to Do?
Here are two approaches to randomize participants:
Approach 1: Using a Single Study
This approach uses features within one Avicenna study to assign groups. It supports Simple randomization but not complex techniques like Block or Stratified. Learn more about randomization types here.
You can implement this using either survey questions or participant variables.
Option 1.1: Using Survey Questions
-
Create a baseline survey: This survey will capture the participant's assigned group. See How to Create a Baseline Survey.
-
Add a single-answer question: Use a single-answer question to represent the group assignment. Avoid number questions as some numbers might be chosen more often than others intuitively.
-
Configure the question for arms:
- Set question content: Write
Choose the option below. - Define answer options: Use identical text (e.g.,
Aor spaces) for all options. Remember the ID-to-arm mapping. Image answers are also possible. - Enable randomization: Turn on
Answer Randomization. Select all answer IDs forIncluded Answer ID(s)and setSelection Countto1.
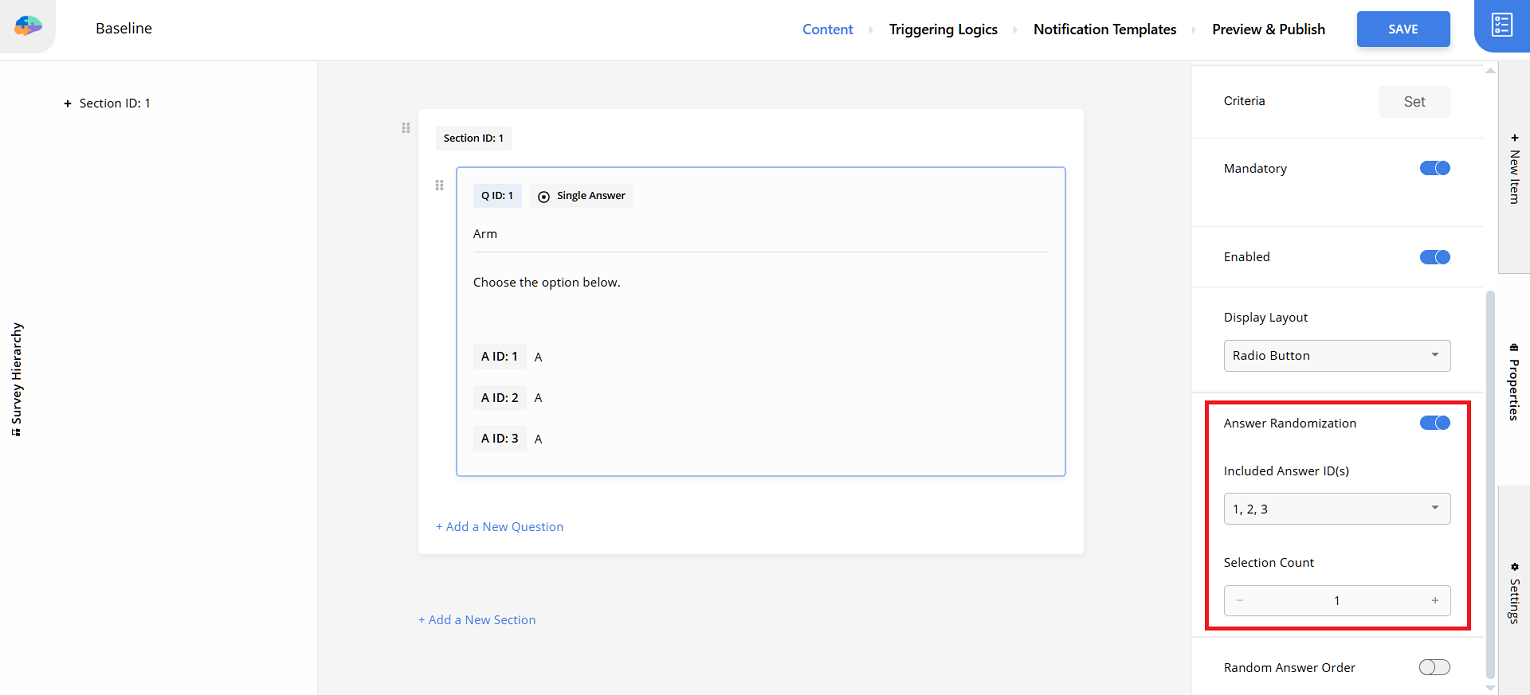
- Set question content: Write
-
Label the question: Use a clear label, like
Arm. -
Make it mandatory: Mark the question as
Mandatory. -
(Optional) Add more questions: Include other necessary baseline questions in the survey.
-
Use the group in criteria: Apply the
Armquestion's response in criteria for activities, triggering logics, or notification templates to customize the experience per arm. Using this question as a placeholder is not recommended as it shows identical text. -
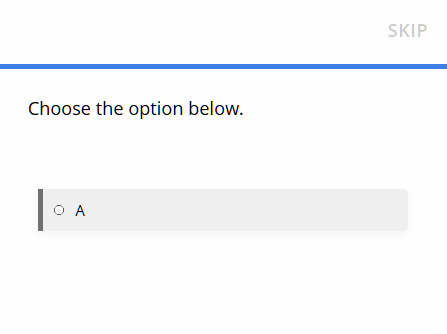
Participant experience: When participants join, they complete the baseline survey. They will see one randomly selected option to determine their group.
Option 1.2: Using Participant Variables
-
Define a participant variable: Treat the participant's arm/group as a variable. Define and set this variable when a participant joins.
-
Assign the variable value: You can either:
- Randomize externally: Perform randomization outside Avicenna (e.g., using a spreadsheet) and set the variable for each participant accordingly.
- Randomize internally: Use the survey method described in Option 1.1 to determine the group and set the variable based on the response. This has the same limitations regarding randomization techniques.
The first approach (using one study) works best if your study arms differ only in their activities and notification templates, not in aspects like data sources.
Approach 2: Using Multiple Studies
This approach involves creating a separate study for each arm.
- Create studies per arm: Set up distinct studies for each arm (e.g., "Control Study", "Intervention Study"). See Create a New Study.
- Use identical consent forms: Ensure all studies use the same Consent Materials. This prevents participants from noticing differences if they have study IDs or registration URLs.
- Randomize externally: Assign participants to arms outside Avicenna (e.g., using spreadsheet randomization).
- Enroll participants: Direct participants to join the study corresponding to their assigned arm, or invite them via email.
Using multiple studies means duplicating the study setup. Common protocols require repetitive implementation. Mid-study changes must be applied across all study copies, though some tools might help streamline this.
Pro Tips
Blinding Considerations:
- Single-blinded studies: Where participants are unaware of their group, the approaches described work well.
- Double-blinded studies: Where researchers also shouldn't know group assignments, use these approaches but restrict access to data revealing group allocation (like baseline survey responses). Manage this using Roles and Permissions.
Choose the approach that best fits your study's complexity and operational workflow.